US regulators approved an HIV drug described as a “miracle” by the UN for its ability to prevent infections with two doses a year.
When given to people at risk of HIV in the US and Africa, Gilead’s lenacapavir vastly outperformed the standard pre-exposure prophylaxis drug, which requires taking several pills daily.
The drug works by stopping the virus infecting cells and replicating within them, and has previously been approved for use as a post-infection treatment, but is so long-acting that it can work preventatively even if only injected every six months.
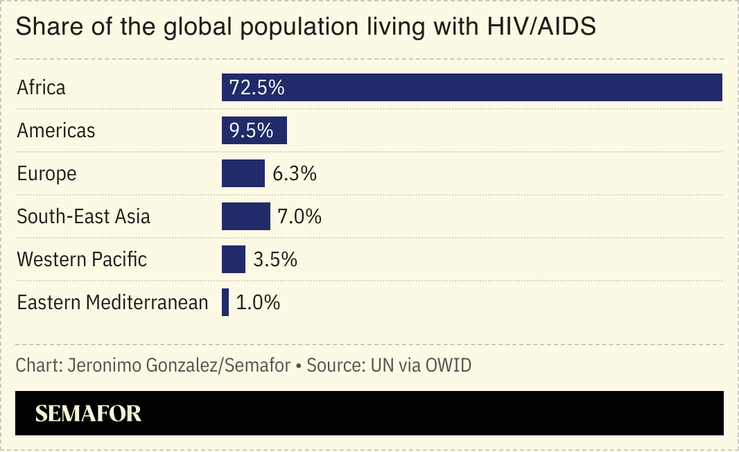
Gilead’s CEO said it could “end the HIV epidemic once and for all.” Almost 40 million people are living with HIV worldwide, with two-thirds of cases in Africa.