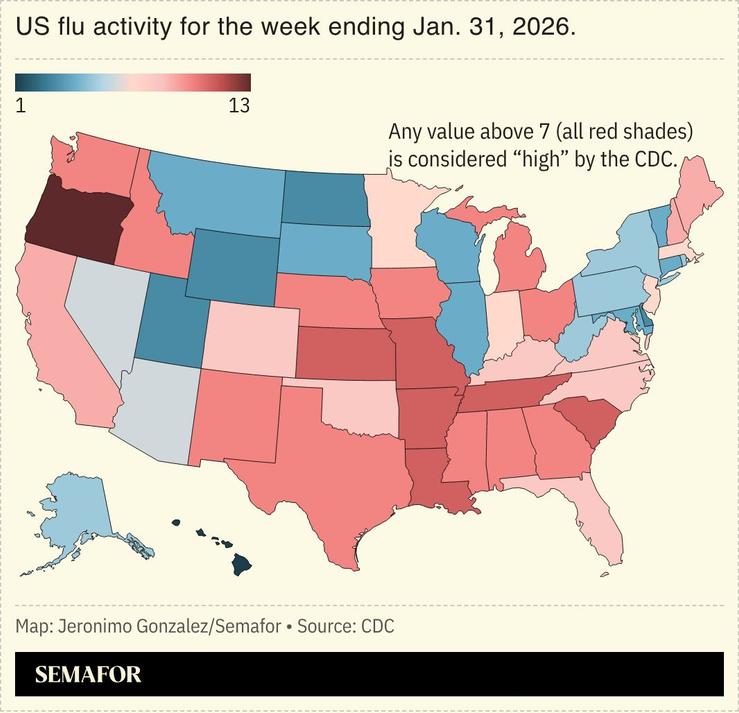
The US medical regulator denied Moderna’s application for a review of its first mRNA flu vaccine.
The US pharma firm produced successful mRNA COVID-19 vaccines, and its 40,000-person study suggested its new shot was about 27% more effective than a standard flu vaccine. But the Food and Drug Administration said the study lacked adequate controls, which Moderna denied.
Such refusals are rare, especially since the FDA had not previously raised concerns, but this White House is vaccine-skeptical, despite the efficacy of Moderna’s COVID vaccine during the first Trump administration.
In another pandemic-era throwback, the federal government is also funding research into whether the deworming drug ivermectin, hailed by some fringe groups as a COVID wonder drug, can treat cancer.